Many people have heard that the batteries powered vehicles. The batteries used in such vehicles, generally, are divided into two categories one is made by Ternary Lithium and other is using Lithium iron phosphate. So, what are the differences in performance between these two different materials? What caused these differences? The field of lithium iron phosphate batteries in the past has begun now turn in to large-scale development of ternary lithium. What is the reason for this?
The structural principle of lithium battery

If you have seen a car lithium battery cell, you must know that its appearance is similar to our usual AA or AA batteries but the size is slightly larger and different in shape. We can see the positive and negative terminals of the battery and the outer casing of the battery from the outside. But what are the basic structure and working principle works inside the battery?
The inside of the battery is mainly composed of three components:
- Positive electrodes generally meant of metal oxide such as lithium cobalt oxide (LiCoO2), however, in newer batteries, you will see lithium iron phosphate (LiFePO4).
- Second is a negative electrode made from carbon -graphite
- The last one is an electrolyte which is lithium salt in an organic solvent, Nickel-cadmium (NiCd), Nickel-metal-hydride (NiMH) or different from battery to battery.
All these materials are packaged in a cylindrical steel cylinder. Since there are many tiny holes in the positive electrode material, a large number of electrons can be stored. During the discharge process, these electrons move through the external circuit to the positive electrode material. When the battery is connected to the external appliance, a circuit from the positive electrode to the consumer to the negative electrode is formed. This is the basic working principle of the battery.
When we charge the battery, the positive electrode starts giving up its lithium ions which travel towards the negative electrode (graphite) and remain there. In this way, the battery store the energy when you charge the battery. On the other hand, when you start using the battery which is the discharging process, during this time the lithium ions start moving back to the positive electrode through the electrolyte but in both the cases the electrons always move to towards the opposite side of the lithium ions via external circuit; they don’t flow through the electrolyte which acts as a barrier for electrons. When the battery is connected to the external appliance, a circuit from the positive electrode to the consumer to the negative electrode is formed. This is the basic working principle of the battery.
If you disconnect the externally connected appliance to the battery, it will stop discharging because the flow of electrons has stopped and so does the movement of the ions through the electrolyte. And the same case when your battery gets discharged, the flow ions has stopped which indirectly also cut off the supply of electrons and you not able to use the device the running on the battery.
For the safety of lithium ions because it recharging capability it comes with an inbuilt controller to control the way of charging. It avoids overcharging and overheating that sometimes cause exploding of the batteries.
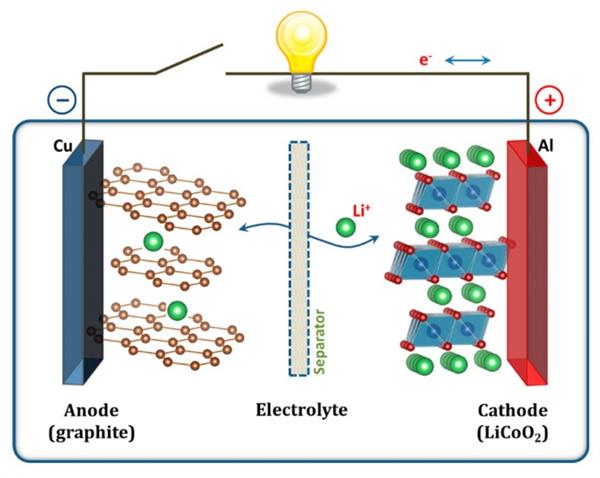
The negative electrode material is actually the graphite we are very common. Since graphite naturally has many pores and is highly conductive, its capacity to capture electrons greatly exceeds that of the positive electrode material. So for lithium batteries, the main bottleneck is stuck on the cathode material. This is why major battery companies are constantly improving the performance of cathode materials in order to increase the charge capacity (energy density) of lithium batteries. We often say that ternary lithium and lithium iron phosphate refer to positive electrode materials.
In the field of conventional lead-acid batteries, the electrolyte is made of dilute sulfuric acid which is extremely low in cost. This is also why we sometimes need to fill the new battery with a liquid when it is first used. This liquid is the electrolyte. Since the main component of dilute sulfuric acid is water, water can be electrically conductive under the action of acidic particles, so that a current loop can be formed inside the battery. However, due to the poor conductivity of water, the voltage of traditional lead-acid batteries is less than 2 volts, and the lithium batteries we use can reach a voltage of 3-4 volts for a single cell, so lithium salts are needed to replace traditional water-based electrolysis ( liquid).
You might be thinking why the electrons do not move back to the positive electrode via electrolyte material, it is because the free electrons need a metallic circuit to travel. Now your electric appliance gives them a metallic circuit to move them without shorting the positive and negative electrodes.
Lithium battery charging and discharging principle
The reason why lithium batteries can be charged and discharged is the process of converting electrical energy into chemical energy during charging and converting chemical energy into electrical energy during discharging. Simply put, lithium ions are inserted into the negative electrode material from the positive electrode material through the electrolyte during charging; lithium ions are inserted into the positive electrode material from the negative electrode material through the electrolyte during discharge, and electrons pass through an external circuit to form a current. For lithium-ion batteries, the performance of the cathode material is critical, it must have good electrical conductivity, good compatibility with the electrolyte and excellent stability.
The performance difference between lithium iron phosphate and ternary lithium
Lithium iron phosphate batteries have high safety and long cycle life. It has been experimentally verified that after 1600 charge and discharge cycles, the lithium iron phosphate battery can still have 80% of the charge.
Not only that, the performance of lithium iron phosphate battery is very stable during high-power discharge. This is a usage scenario that we often encounter: in the case of rapid acceleration or high speed, the battery needs high-power discharge, and in this process, the stability of the voltage can make the vehicle have better performance. Moreover, the actual capacity of the battery will decrease during the high-power discharge process, which is one of the reasons why the high-speed cruising range of the electric vehicle will be reduced, and the stability of the lithium iron phosphate battery is very prominent.
Battery pack and motor assembly for pure electric vehicles
Another advantage of lithium iron phosphate is its high safety. It can withstand high temperatures of 700-800 degrees. It will not release oxygen molecules for extreme conditions such as puncture, impact, and short circuit, so it will not burn and explode. This is also why still lithium iron phosphate batteries used in vehicles with a large number because the safety requirements are higher, moreover, they are not so sensitive to weight and volume, so lithium iron phosphate is a very good choice.
However, lithium iron phosphate also has two disadvantages that cannot be ignored: the first is that the energy density is relatively low, the weight is large and the volume is large; the second is relatively sensitive to temperature, and the charged amount will decrease significantly in the low-temperature environment.
The are two main elements the ternary battery, namely nickel-cobalt-manganese and nickel-cobalt-aluminum, among which the combination of three elements of nickel-cobalt-manganese is the most representative. It combines the advantages of lithium cobaltate, lithium nickelate, and lithium manganate, and actually combines the three materials together. Therefore, its comprehensive performance is better than a single material, and its energy density can exceed 200 watt-hours/kg, so the energy density is the biggest advantage of ternary lithium.
However, contrary to lithium iron phosphate, ternary lithium is less safe and has a maximum temperature tolerance of 250-300 degrees. Therefore, in the event of impact or puncture, it is easy to cause a fire and explosion. Therefore, the cooling system, as well as the thermal management system of the entire battery pack, requires higher cost development and design.
Including the Accord and Camry hybrid versions of Japanese manufacturers, the latest models are also using ternary lithium batteries. After using ternary lithium, the energy density is significantly improved, the battery volume is reduced, and the weight is reduced, which provides more practical space for the passenger compartment or the trunk. Therefore, on the whole, the ternary lithium battery has a more obvious advantage in the current policy environment.
Via or Source: diandong
Related Posts
What are Nuclear Batteries? Their types, advantages & disadvatnages
Trending Smartphones of 2020 having Gigantic Battery Backup
AMANI Launches 4000mAh Mobile Battery-Mi BM 47/MI3S for MI Handsets
Paper battery vs Conventional Battery- Difference
Performance difference between lithium iron phosphate and ternary lithium
Why mobile phones heating up and draining battery? How to solve this problem?